Fruit ripening|Fruit ripening hormone
Reproduction in flowering plants begins with the formation of the flower and ends with the formation fruit and seeds. Fruit development and seed set in flowering plants normally occur in coordinated manner following pollination of the stigma and subsequent double fertilization in the ovule, a female gamete forming structure, located with the carpel. When the egg and central cell of the female gametophyte are not fused with sperm cells, they remain in quiescent state and eventually degrade as the flower undergoes senescence. This has led to the interpretation that signaling processes are required to activate development of the fertilization products leading to the initiation of seed and fruit development.
Fruit development can be uncoupled from fertilization in plants that undergo the genetically controlled process of parthenocarpy and apomixes. Apomictic species can produce both fruit and viable seed in absence of fertilization.
Fruit development can be uncoupled from fertilization in plants that undergo the genetically controlled process of parthenocarpy and apomixes. Apomictic species can produce both fruit and viable seed in absence of fertilization.
Parthenocarpy has a genetic basis and has been exploited by farmers and plant breeders for the production of seedless fruits.
Most fruits develop from a gynoecium’s that contains one or more carpels. In pseudocarpic fruit, organs other than the gynoecium (e.g. receptacl bract, the floral tube, or the enlarged axis of the inflorescence) participate in the formation of fruit.
When an ovary develops into a fruit, the ovary wall becomes the pericarp. Fruit, protects seed development and server the vehicle for seed dispersal. Fruits also provide human with a source of nutrition, culinary diversity, and often great pleasure. Fruits from domesticated species often have been tremendously enlarged over that normally found in the progenitor wild species.
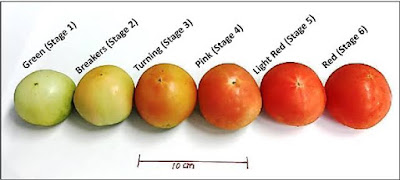
Initially, fruit enlarge through cell division and than by increasing volume. The embryo matures and the seed accumulates storage products, acquires desiccation tolerance, and less water. The fruit then ripens Ripening is accompanied by changes in aroma, color, flavor, nutritional contents, and susceptibility to opportunistic pathogens. Ripening can be generally defined as the summation of changes in tissue metabolism rendering the fruit organ attractive for consumption by organisms that assist in seed release and dispersal. The ripening renders, the fruit attractive (appealing) to organisms receiving sustenance in exchange for assisting in seed dispersal.
Ripening physiology has been classically defined as, either ‘climacteric’ or ‘non climacteric’. Climacteric fruits show sudden increase in respiration at the onset of ripening, usually in concert with increased production of ethylene. Ethylene is typically necessary for climacteric ripening. Non climacteric fruits do not increase respiration at ripening and often have no requirement for ethylene to complete maturation. Fruit growth, in most species, can be represented by sigmoidal curve. Physiologically and biochemically, fruit development can be divided into four phases, which although occur in continuity, are separated on the basis of the major activities. Phase I includes ovary development in the flower, and (following anthesis) a decision to abort or proceed with further development. Phase II involves a period of rapid cell divisions. Phase III is the period of most rapid growth, when cell divisions (more or less) ceases and growth is almost exclusively by cell enlargement.
During this phase, food reserves are accumulated and most fruits attain their final shape and size before the onset of ripening Phase IV. In some plant species another burst of growth occurs during the ripening period (and hence fruit of such types exhibit double sigmoid growth curve). In some fruits, such as avocado, cell divisions continue well into Phase III. There is much variation among flowering plants in the manner in which fruit arise. Fruit may arise from single or multiple fused ovaries of a single flower (simple fruits), from several single ovaries of a single flower (aggregate fruits), or from ovaries of several flowers in inflorescence multiple fruits.
Mature fruits can be generally characterized as either fleshy or dry. Fleshy fruits typically undergo ripening as defined above and dry fruits (cereals and legumes) mature in a process more akin to senescence and disperse their seeds via abscission like programs, including dehiscence or shattering.
Fruit ripening is a unique aspect of plant development with direct implication for large components of food supply and related areas of human health and nutrition. The ripening of fruit organs represent the terminal stage of development in which the matured seeds are released. Although the specific biochemical and physiological programs resulting in ripening phenomena vary among species, typical changes include (a) alteration in chlorophyll and carotenoid contents and accumulation of flavonoids resulting in modification of color, (b) alteration of cell turgor and cell wall structure and/or metabolism and change in texture, (c) modification of starch sugars, acids, and volatile profiles that affect nutritional quality, flavor, aroma and taste. From a practical viewpoint, a number of ripening characteristics result in negative quality attributes including decrease self–life and high input harvest, shipping/transport and storage practices. The ripening associated changes in firmness and overall decrease in resistance to microbial infection brought about by the ripening process and associated tissue deterioration are particularly important. Ripening has an impact on fiber content and composition, lipid metabolism, and levels of vitamins and various antioxidants. It is important to understand the process of fruit ripening as this will increase the ability to understand and manipulate, through breeding or biotechnology, key control points in the global control of ripening or specific stages/regulatory points of specific ripening process.
Climacteric fruit typically increase biosynthesis of the gaseous hormone ethylene, and display a burst respiration at the onset of ripening. Fruit such as apple, banana, pears and tomato exhibit climacteric fruit ripening. Interestingly climacteric fruit span a wide range of angiosperms both dicots and monocot. Non-climacteric fruit, including strawberries, grapes and citrus fruit do not require climacteric respiration or increased ethylene for maturation.
Mature fruits can be generally characterized as either fleshy or dry. Fleshy fruits typically undergo ripening as defined above and dry fruits (cereals and legumes) mature in a process more akin to senescence and disperse their seeds via abscission like programs, including dehiscence or shattering.
Fruit ripening is a unique aspect of plant development with direct implication for large components of food supply and related areas of human health and nutrition. The ripening of fruit organs represent the terminal stage of development in which the matured seeds are released. Although the specific biochemical and physiological programs resulting in ripening phenomena vary among species, typical changes include (a) alteration in chlorophyll and carotenoid contents and accumulation of flavonoids resulting in modification of color, (b) alteration of cell turgor and cell wall structure and/or metabolism and change in texture, (c) modification of starch sugars, acids, and volatile profiles that affect nutritional quality, flavor, aroma and taste. From a practical viewpoint, a number of ripening characteristics result in negative quality attributes including decrease self–life and high input harvest, shipping/transport and storage practices. The ripening associated changes in firmness and overall decrease in resistance to microbial infection brought about by the ripening process and associated tissue deterioration are particularly important. Ripening has an impact on fiber content and composition, lipid metabolism, and levels of vitamins and various antioxidants. It is important to understand the process of fruit ripening as this will increase the ability to understand and manipulate, through breeding or biotechnology, key control points in the global control of ripening or specific stages/regulatory points of specific ripening process.
Climacteric fruit typically increase biosynthesis of the gaseous hormone ethylene, and display a burst respiration at the onset of ripening. Fruit such as apple, banana, pears and tomato exhibit climacteric fruit ripening. Interestingly climacteric fruit span a wide range of angiosperms both dicots and monocot. Non-climacteric fruit, including strawberries, grapes and citrus fruit do not require climacteric respiration or increased ethylene for maturation.
Surprisingly members of same (e.g. melon) or closely related (e.g. melon and water melon) species are reported to include both climacteric and non-climacteric types. Genetic and molecular distinction between climacteric and non-climacteric fruit ripening are poorly understood.
Nevertheless, it seems likely that non climacteric phenotypes may represent mutations in ethylene synthesis or signaling as opposed to more complex distinction.
Several mutants (spontaneous and induced) that affect fruit development and ripening have been identified and characterized particularly in tomato climacteric fruit).
Ripening in Climacteric fruits
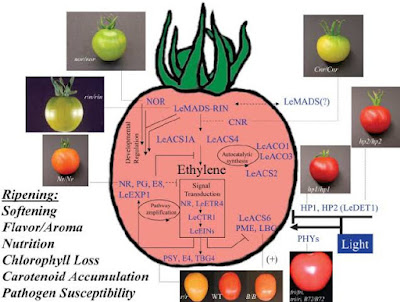
Apple, Avocado, Banana, Cantalope, Mango, Olive, Papaya, Passion fruit, Peach, Pear, Plum and tomato are common climacteric fruits. These are also import commercial crops. In these fruits ethylene acts as an inducer of many genes associated with color change, changes in carbohydrate reserves, and softening of pulp. Ripening of climacteric fruit is induced by ethylene. It seems justified to assume that other factors that operate prior to induction of ethylene biosynthesis also control early developmental stages of ripening fruit.
Transgenic tomatoes, where, ethylene production is drastically curtailed by inserting the coding sequence of the ACC Synthase or the ACC oxidase gene in an antisense orientation, under control of strong promoter CaMV S 35,show no climacteric and do not ripen.
Ethylene production in plants results from methionine metabolism. The rate–limiting steps in fruit ethylene synthesis include the S–adenosylmethionine (SAM) to 1 aminocyclopropane–1–carboxylic acid (ACC) via ACC synthase (ACS) and the subsequent metabolism of ACC to ethylene by ACC oxidase (ACO). Both the ACC synthase and the ACC oxidase are encoded by multigene families. Anti-sense repression of ACS and ACO genes has clarified the role of these genes in regulating climacteric ethylene synthesis. It is also well known, however, that ethylene alone is not sufficient for ripening and that a developmental “competence” to responds to ethylene must be achieved.
This is obvious from the fact that immature fruit typically do not ripen in response to exogenous ethylene. Climacteric fruit ripening is regulated by developmental factors that must properly coordinate with ethylene synthesis. These include colorless non-ripening (Chr) ripening inhibitor (rin) and non–ripening (nor).
Valuable information has been generated through studies on developmental mutations that affect all aspects of the fruit ripening processes at least in tomato. These include colorless non–ripening (Chr), ripening inhibitor (rin),non–ripening (nor).
Figure 24-Fruit ripening mutants of Tomato (from left to right ,ripe fruit of wild type ,ripening inhibitor(rin), non-ripening(nor), Never-ripe(Nr)and Green-ripe(Gr).
In these mutants fruit ripening is severely impaired. These do not undergo an increase in ripening–related ethylene production and show inhibition of ripening related gene expression. In these mutants, however expression of ripening–related genes is partially restored but not ripening by application of ethylene. Thus these mutants remain ethylene responsive.
Although a complete molecular biological and signal transduction pathway remain to be identified/defined. An important first step towards this goal came from the discovery that the rik locus encodes a MADS–box transcription factor necessary for regulating ripening. In other two mutants of tomato Green–ripe (Gr), and Never–ripe (Nr) studies have indicated that their reduced ripening results from decreased ethylene sensitivity.
Light has been shown to affect carotenoid accumulation in plants. It has been shown that phytochrome mediated light signal transduction is required for normal ripe fruit pigmentation but it not affect other ripening attributes.
The change from a green fruit to ripe apple, banana and tomato involved a transition of chloroplasts to chromoplasts. Chlorophyll pigments and thylakoid membranes are broken down. A progressive accumulation of new carotenoid pigments occur in the plastids. The new carotenoids in tomato include β–carotene and lycopene.
These provide the orange and red color for the ripe fruit respectively. Lycopene synthesis is induced by ethylene, and is shutt off by inhibitors of ethylene synthesis or perception. Fruit of Banana, Kiwi fruit and mango synthesize and accumulate starch during development. During ripening these fruits hydrolyze starch and convert it into sugars. Fruits of Melon like systems continuously import sugars from other parts of the plant. During ripening, the fruits show an increase in the concentrations of sugars.
Moreover, sucrose is hydrolyzed to hexoses. Ethylene–induced upregulation of mRNAs of enzymes involved in starch/sugar metabolism (such as sucrose phosphate synthase and acid invertase) occur during ripening in several fruits. These events change sweetness of fruit.
Production of organic acids (principally citric acid but also malic acid) and volatiles increase while fruits ripen.
These combine to produce the unique flavor and aroma. Production of phenolics, provide astringency to unripe fruits. These have profound influence on the flavor and color of mature fruits.
Fruit texture changes drastically during ripening. The cell walls are degraded by increased activities of various hydrolyzing enzymes. Distruption of the cellulose hemicellulose and petin networks is done by the increased activities of enzymes. The wall–loosening enzymes, such as endo–1, 4 β–glucanases (E Gases) and xyloglucan endotransglycosylases (XETs), are activated. Genes encoding expansive and XETs, and E Gases expressed during fruit ripening are induced by ethylene and down regulated by auxin. Activities of enzymes; Pectin methylesterase (PME) ad polygalacturonase (PG) are enhanced during ripening fruits.
These affect large changes in pectin structure. The production of PG is under developmental control. The engyme is absent in green fruits and is synthesized de novo during ripening. It production is controlled by ethylene. The activities of stress/pathogen inducible enzymes, superoxide dismutase (SOD) and catalase occur in ripening fruit. Both of these are known for antioxidant activities.
Ripening and non-climacteric fruits:
Strawberry has been studied among the non-climacteric fruits. Majority of the changes that occur during ripening of non climacteric fruits are similar to the changes that are seen in ripening of climacteric fruits.
Ripening of non-climacteric fruits (at least in strawberry) is not affected by exogenous ethylene and inhibitors of ethylene biosynthesis and ethylene response/action. Auxins retard/inhibit ripening related changes in strawberry.
Grape berry and citrus fruits are two other non-climacteric fruits. Treatment with auxin retards ripening of these fruits. Citrus fruit is unusual; it is non climacteric fruit, but carotenoid synthesis in the orange peel is regulated by ethylene.
Abscission of mature/ripe fruit, similar to leaves and flower pedicels is regulated by ethylene. Abscission is inhibited by auxins, cytokinins and cytokins.














0 Comments
If you have any query let me know.