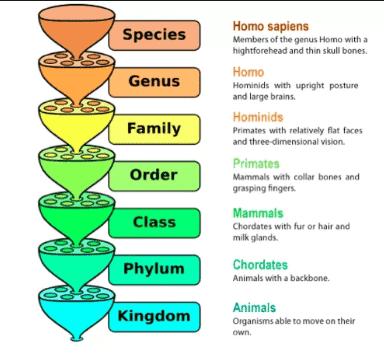
Diffusion is the movement of molecules of any substance from a region of it’s higher to a region of it’s lower concentration (down its own concentration gradient) to spread uniformly in the dispersing phase on account of their random kinetic motion.
 |
| Source Wikipedia |
The rate of diffusion is directly proportional to: 1. the concentration of the substance 2. temperature of the medium 3. area of the diffusion pathway The diffusion is inversely proportional to: 1. the size of the substance molecules 2. the molecular weight of the substance molecule 3. the distance over which the molecules have to diffuseDiffusion through Biomembranes
Gases and little hydrophobic molecules diffuse directly across the phospholipid bilayer at a rate proportional to their ability to dissolve during a liquid hydro carbon. Transport of molecules takes place along the concentration gradient and no metabolic energy is expended in this process. This can be described as ‘down hill transport’. Diffusion through the bio membrane takes place in two ways. 1. Diffusion of fat-soluble substances through plasma membrane simply by dissolving in the lipid bilayer. 2. Diffusion of water soluble substances and ions: This takes place through pores in the membranes. Diffusion of charged particles water soluble substances and ions such as K+ Cl– and HCO3– diffuse through the pores in the membranes. An ion diffuses from the side richer in like charges to the side with an excess of opposite charges. The difference of electrical charges between the two sides of a membrane is called electric chemical gradient.













0 Comments
If you have any query let me know.